Intracellular Transport of Neuronal Resources and Cellular Function:
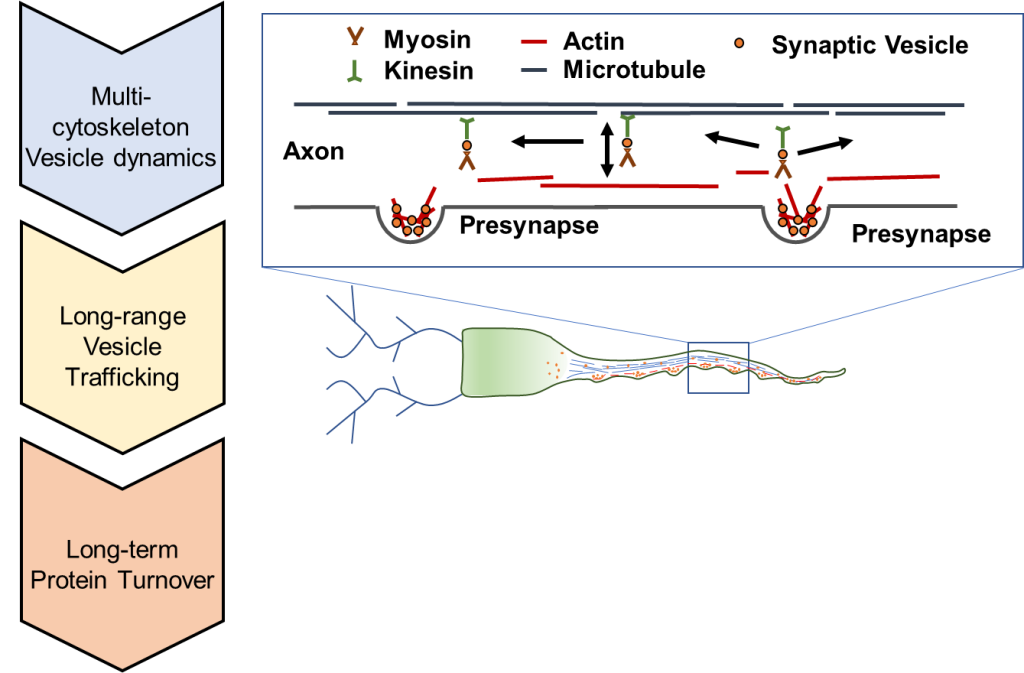
How do resources navigate the roadways between synapses in a neuron? This research area focuses on the mechanisms and mechanics of intracellular transport. We utilize the latest high-resolution fluorescence microscopy techniques in live-cell imaging. These methods allow us to track individual resources, such as synaptic vesicles, at the nanometer and millisecond scales.
1) Experimental Fluorescence Microscopy Approach:

Our lab utilizes high-resolution fluorescence microscopy to label and track synaptic vesicles at the nanometer and millisecond scales. We also co-label single vesicles with other proteins labeled fluorescently using different colors. We then use these results to distinguish how vesicle trafficking correlates with synapse structure/function as well as cellular structure/function (see left).
2) Computational Simulation Approach:

Our lab develops computational simulations of vesicle trafficking from the molecular to cellular level (as shown above). We use these simulations to connect the experimentally measured vesicle mechanics at the molecular level to cellular function and the cellular scale. We then use these simulations to probe the limits of molecular processes to determine their effects on cellular physiology.
3) Experimental Data Computational Analysis Techniques:
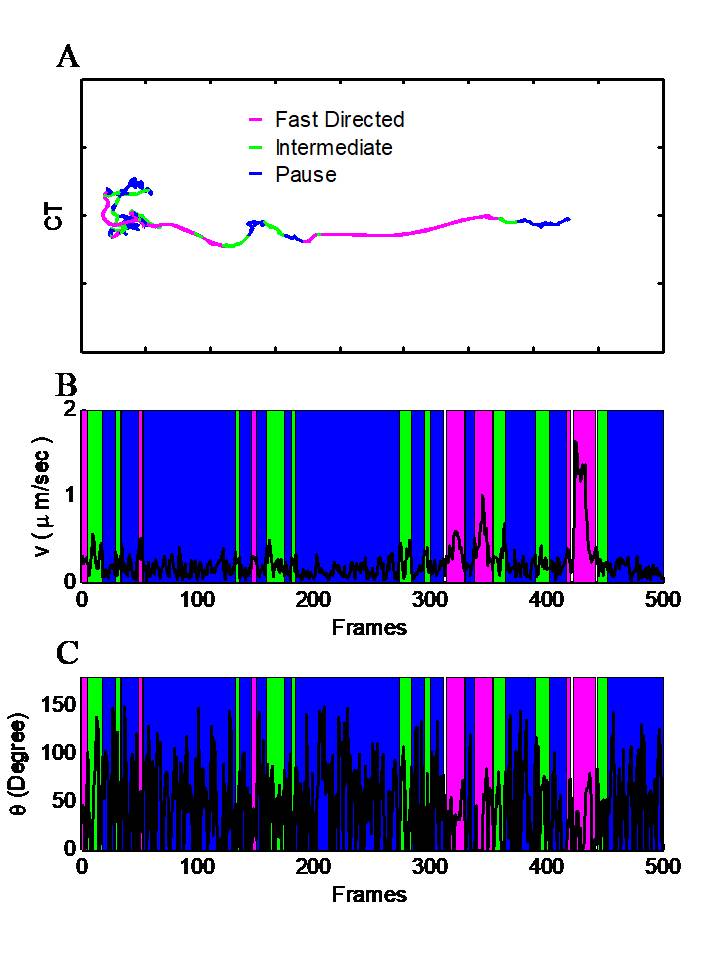
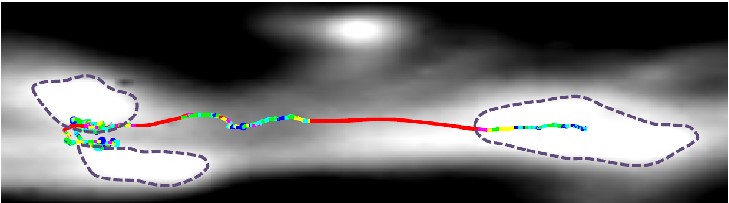
Our lab creates and applies advanced computational tools to quantitatively understand mobility of resources inside live functioning neurons. We apply the traditional physics paradigm to neuroscience observations. We analyze our experimentally obtained high-precision microscopy images. We quantify the type and nature of motion observed inside the cell. We then leverage our quantitative results to distinguish the physical mechanics of individual processes involved in intracellular transport. With these insights we seek to observe to physical phenomena important to understand neuroscience problems.
The role of molecular mechanics on vesicle mobility.
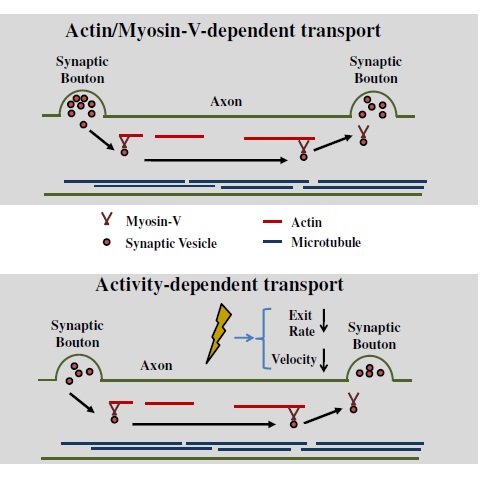
Our lab explores the mechanics of vesicle mobility at the molecular level. Efficient transport of resources throughout the cell requires a complex combination of interacting proteins. In particular, we explore motor proteins that drive vesicle mobility (such as Myosin-V). We explore the road-ways that support transport (such as actin, or microtubules).
We also explore the fundamental effects of activity on efficient cellular transport. Activity is the main pathway a neuronal synapse is triggered to transmit information to the next neuron. We explore the mechanics of how activity alters the mechanics of individual proteins, in turn, resulting in information transfer.